HIGH RESOLUTION MASS SPECTROMETRY
Mass spectrometry (MS) is an analytical technique that measures the mass-to-charge ratio of charged particles.
- Molecular weight determination
- Structural characterization
- Qualitative and quantitative analysis of components in a mixture.
Areas of applications:
- Proteomics
- Pharmaceutical analysis
- Forensic analysis
- Environmental analysis
- Clinical applications
ORBITRAP MASS SPECTROMETRY
Orbitrap mass spectrometers deliver a total possible maximum resolution (FWHM) of 1,000,000 at m/z 200 and a sub-1 ppm mass accuracy in a single compact and easy-to-use instrument. These high-resolution accurate-mass systems detect a wide range of compounds and small molecules during both targeted and untargeted analyses, without losing selectivity or sensitivity. Simply put, when it comes to Orbitrap technology, there is no compromise.
High Resolution Mass Spectrometer (HRMS) AT CBA FACILITY
Make: ThermoFisher Scientific
Model: Q Exactive Plus Biopharma Orbitrap Mass Spectrometer

- Resolution up to 240,000 (FWHM) at m/z 200
- Analysis of large molecules up to m/z 8000
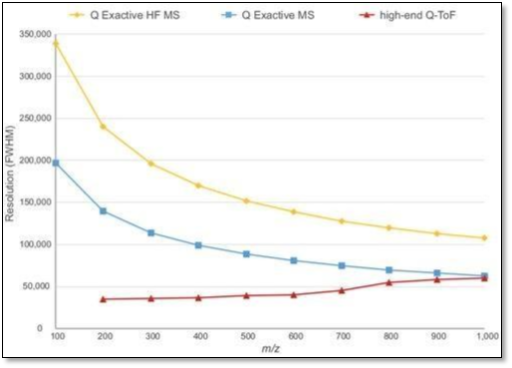
Figure 1. The different resolution achievements of Orbitrap instruments compared to Q-TOF mass spectrometers.
Glycan Analysis
Glycan analysis is one of the critical quality attribute in analytical characterization of proteins.
Glycosylation is an important PTM as it has a great impact on the safety, efficacy and pharmacokinetics of the protein drugs.
Glycosylation can be classified as N-linked glycosylation (Asn residue through the Asn-X-Thr/Ser consensus sequence) or O-linked glycosylation (serine or threonine residues).
N-glycosylation of protein therapeutics is characterized at different levels including released glycans, glycopeptides, glycosylated protein subunits, and intact glycoproteins.
Advancements in separation techniques and mass spectrometry have been successfully employed for glycan analysis at different levels, including released glycan, glycopeptide, glycosylated protein subunit and intact protein levels.
GUIDANCE FOR INDUSTRY
EMA guideline states:
- “The carbohydrate content (neutral sugars, amino sugars and sialic acids) should be determined. In addition, the structure of the carbohydrate chains, the oligosaccharide pattern (antennary profile), the glycosylation site(s) and occupancy should be analysed.
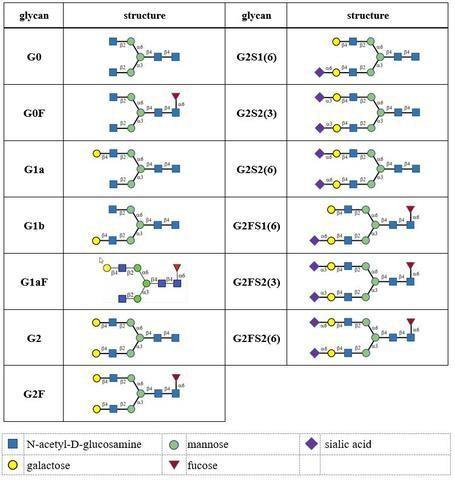
- Typically, monoclonal antibodies have one N-glycosylation site on each heavy chain located in the Fc region. The light chain is usually not glycosylated. However, additional glycosylation site(s) in the heavy chains may occur, and thus their presence or absence should be confirmed. Glycan structures should be characterised, and particular attention should be paid to their degree of mannosylation, galactosylation, fucosylation and sialylation. The distribution of the main glycan structures present (often G0, G1 and G2) should be determined.
- Higher-order structure of the monoclonal antibody should be characterised by appropriate physicochemical methodologies.“
ICH Q6B guideline states:
“For glycoproteins, the carbohydrate content (neutral sugars, amino sugars, and sialic acids) is determined. In addition, the structure of the carbohydrate chains, the oligosaccharide pattern (antennary profile), and the glycosylation site(s) of the polypeptide chain are analyzed, to the extent possible.”
CASE STUDY OF IgG 1 mAb
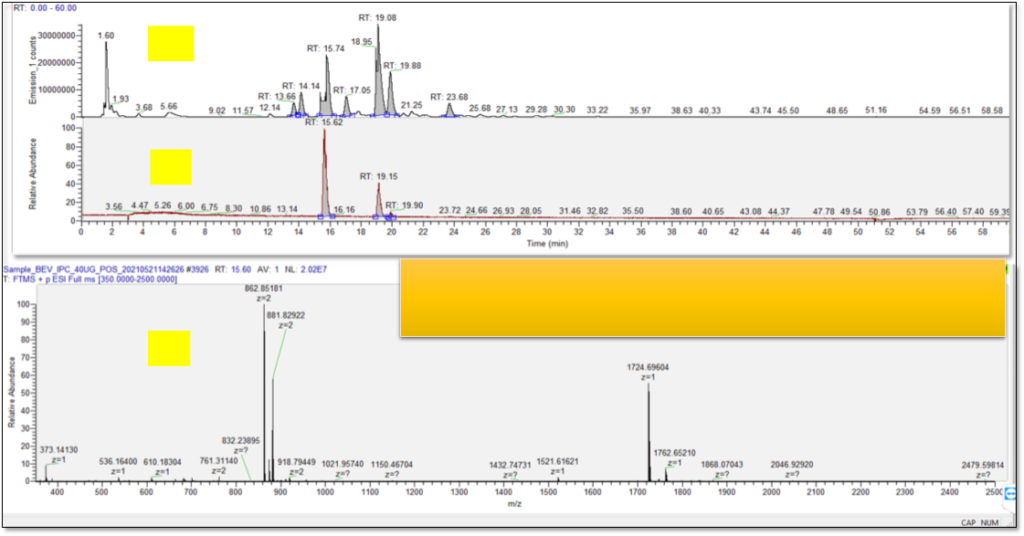
GLYCAN ANALYSIS AT CBA
|
|
Glycan analysis of protein |
| Sample requirement | Amount- 0.1-0.5 ml (10mg/ml) |
| Deliverables | Enzymatic digestion, labeling, Glycan profiling, relative quantification, blank run; QC sample, data report |
| Information required from Client | Expected molecular weight and formula
Sample solubility, polarity and formulation buffer Concentration |
