Biopharma Testing Services
Reach us to better understanding!!

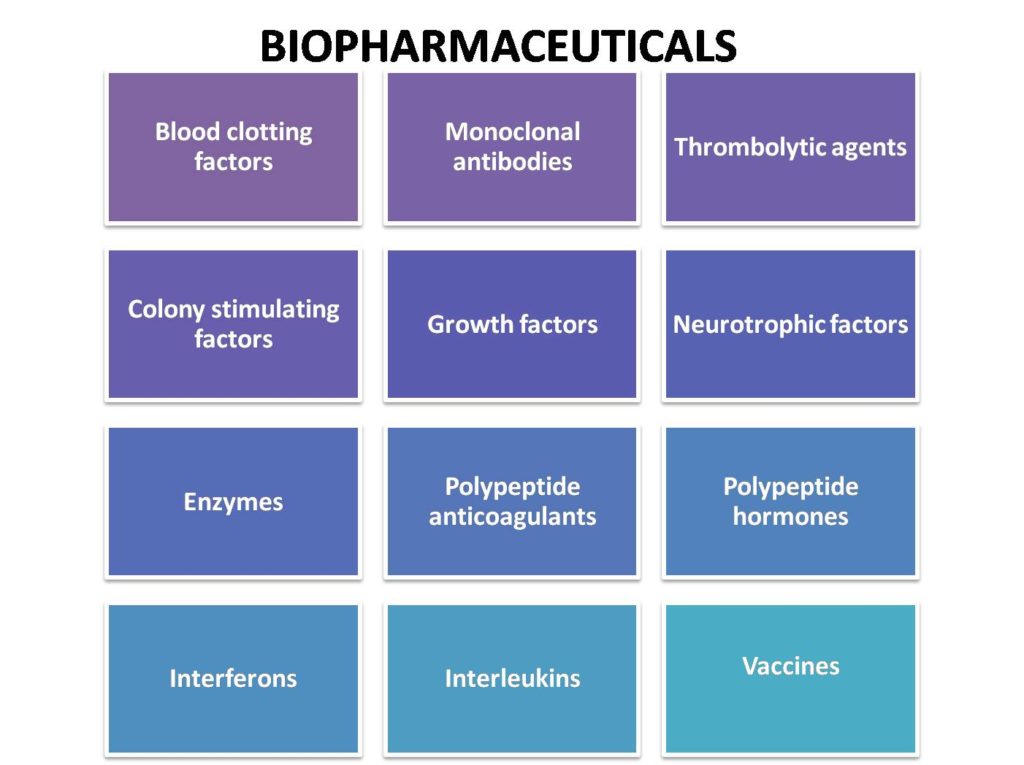
Biopharmaceuticals
Currently, biopharma is the fastest growing and emerging sector in pharma. Many big pharma giants are now shifting their presence to biopharma domain. Biopharmaceuticals are proving to be lifesaving drugs for various dreadful diseases. Biopharmaceuticals are drugs produced using recombinant DNA technology or extracted from living systems. The recombinant human insulin (trade name “Humulin”) was the first biopharmaceutical approved for human therapeutic uses and marketed in 1982. Biopharmaceuticals are now well established as a clinically and commercially important class of therapeutics. They have multiple clinical applications and various advantages for disease therapy, prevention, and diagnosis. Biopharmaceuticals are used for diabetes, dwarfism, myocardial infarction, congestive heart failure, cerebral apoplexy, multiple sclerosis, neutropenia, thrombocytopenia, anaemia, hepatitis, rheumatoid arthritis, asthma, Crohn’s disease and cancers therapies. Biopharmaceuticals can be grouped based on their molecular types that include antibody-based drugs, Fc fusion proteins, anticoagulants, factors, bone morphogenetic proteins, engineered protein scaffolds, enzymes, growth factors, hormones, interferons, interleukins, and thrombolytics.
Comparison: Biopharmaceuticals Vs Pharmaceuticals
Everyone who is planning to initiate any kind of research and development work in area of Biopharmaceuticals, need to very well comprehend the differences between the small molecule pharma drugs and the Biomolecule drugs.
| Pharmaceuticals | Biopharmaceuticals |
| Low molecular weight | High molecular weight |
| Produced by chemical synthesis | Produced by living cells |
| Stable | Sensitive to environmental factors like pH, storage |
| Simple and standard manufacturing processes | Complex and requires specialized manufacturing processes |
| Non immunogenic | Immunogenic |
| Side effects are more as these are less specific | Less side effects as these are more specific |
| Characterization is comparatively easily | Characterization is complex |
Biopharmaceutical drug development process
Extensive analytical characterization is required at each stage of drug development- process and product development phases. It helps gain the confidence that molecule with required right structure will generate the required functional response. In general, SEC, cIEF, UV-Vis spectroscopy, CE-SDS and LC-MS techniques help in early-stage drug development and additional techniques are employed as orthogonal methods during the later stages of drug development.
During Process development, analytical tools are required to analyze the media components, leachable, characterize the different structure levels of drug substance etc. Product development involves extensive characterization of both drug substance and drug product.
The characterization strategy is majorly governed by regulatory guidelines. The developers need to refer ICH Q6B and country specific guidelines like EMEA, US-FDA, CDSCO etc. depending on the region where they plan to launch their product.
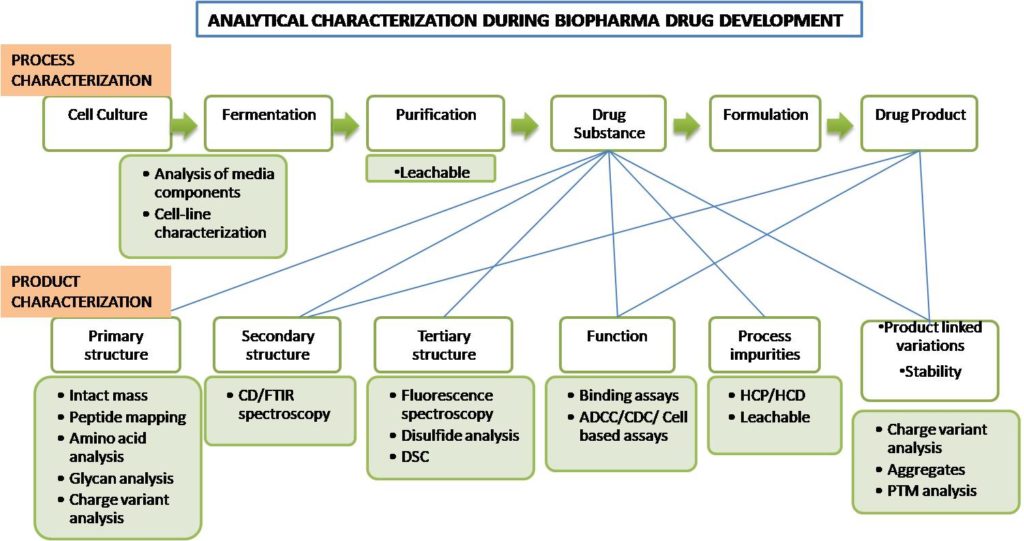
At CBA, we are offering analytical characterization of all types of Biopharmaceuticals at different stages of development to help the Biopharma developers ensure the identity and quality of their product and expedite the development process. For more information about our protein characterization services, visit: https://bioanalysis.in/protein-characterization/
Analytical tools employed for Biopharma testing
Mass spectrometry
Mass spectrometry (MS) is an analytical technique that measures the mass-to-charge ratio of charged particles. MS finds wide applications in the area of Proteomics, Pharmaceutical analysis, Forensic analysis, Environmental analysis and Clinical applications. Mass analysis of intact proteins using high resolution mass spectrometry is a rapid method for confirming the identity and the primary structure of proteins. Mass analysis can provide information for evaluating and comparing the peptide maps, isoforms, glycoforms, and molecule wide modifications associated with a protein. At CBA, we have a Orbitrap HRMS tool for protein characterization. Orbitrap mass spectrometers deliver a total possible maximum resolution (FWHM) of 1,000,000 at m/z 200 and a sub-1 ppm mass accuracy in a single compact and easy-to-use instrument. These high-resolution accurate-mass systems detect a wide range of compounds and small molecules during both targeted and untargeted analyses, without losing selectivity or sensitivity. Simply put, when it comes to Orbitrap technology, there is no compromise.
Surface Plasmon Resonance
Surface Plasmon Resonance (SPR) is an optical biosensor-based technique widely used for studying protein-protein interactions and determining the binding constant and kinetic parameters. It is a label free technique which makes real time measurements. SPR analysis has been used for antibody characterization for more than 20 years. SPR helps in understanding the mechanism of action, target binding and overall efficacy of the protein drug. It is a useful tool during discovery, development and batch release of drugs. At CBA, we have a BIAcore T200 technology to support ligand binding assays.
Chromatography
Size-exclusion chromatography (SEC), ion exchange chromatography (IEX), hydrophobic interaction chromatography (HIC) and reversed phase liquid chromatography (RPLC) are the chromatographic techniques widely employed for biopharma testing. Ion exchange chromatography (IEX) is a powerful technique for the qualitative and quantitative evaluation of charge heterogeneity. SEC separates biomolecules according to their hydrodynamic radius and is a workhorse for aggregation analysis. Hydrophobic interaction chromatography (HIC) is often used for the purification of proteins. The RPLC offers outstanding separation capabilities useful for purity analysis. And it is also used as a front end for the powerful MS detector making LC-MS an indispensable analytical tool for protein characterization. At CBA, we are equipped with a SEC-MALS and UHPLC system.
CD Spectroscopy
Circular Dichroism (CD) is a type of absorption spectroscopy that can provide information on the structures of many types of biological macromolecules It measures the difference between the absorption of left and right-handed circularly-polarized light by proteins. CD is used for protein structure determination, induced structural changes, protein folding/unfolding etc. CD is specifically used for secondary and tertiary structure determination of proteins. We offer secondary, tertiary and temperature dependent structure analysis using CD spectroscopy.
Differential Scanning Calorimetry
Differential Scanning Calorimetry (DSC) measures the amount of heat absorbed or released by dilute in-solution bio-molecules as they are heated or cooled. Macromolecules such as proteins respond to heating or cooling by unfolding at a characteristic temperature. The more intrinsically stable the biomolecule, the higher the midpoint temperature of the unfolding transition. As these processes often exchange microjoule levels of heat, the sensitivity of the Nano DSC is critical for successful investigation of the reaction. The Nano DSC obtains data with less sample than competitive designs and produces unmatched short term noise (±15 nanowatts) and baseline reproducibility (±28 nanowatts). At CBA, we have a Nano-DSC system for the higher order analysis.
Fluorescence spectroscopy
Fluorescence spectroscopy (FLD) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. Fluorescence is a photon emission process that occurs upon molecular relaxation from an electronically excited state to an electronic ground state. Fluorescence spectroscopy is widely used in Biochemical research. “Intrinsic Protein Fluorescence” usually refers to the fluorescence emission of the tryptophan amino acids. This phenomenon is explored for tertiary and higher order structure analysis of proteins using FLD.
FTIR spectroscopy
FTIR, particularly ATR-FTIR has been long used for secondary structure analysis. Amide spectral bands are most commonly used for this protein characterization, particularly the Amide I (1650 cm−1) and Amide II (1545 cm−1) bands. This technique when combined with latest data processing tools can also be explored for studying stability and other CQAs of a protein drug molecule.
Electrophoresis
Electrophoresis is routinely used for separation and analysis of proteins. It is a helpful tool to get insights about protein purity and approximate molecular weight. Advanced forms of electrophoresis like capillary electrophoresis, iso electric focusing are gaining popularity for various applications in protein characterization.
Techniques mentioned above, are a few important analytical tools currently available at CBA for complete characterization and biopharma testing. For more information about technical capabilities, visit: https://bioanalysis.in/facilities/
