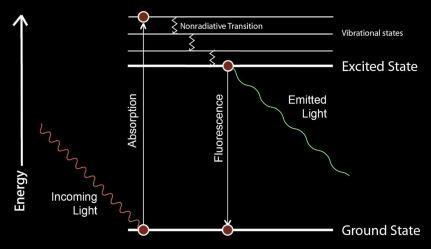
FLUORESCENCE SPECTROSCOPY
Fluorescence spectroscopy (also known as fluorometry or spectrofluorometry) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. Fluorescence is a photon emission process that occurs upon molecular relaxation from an electronically excited state to an electronic ground state. Fluorescence spectroscopy is widely used in Biochemical research.
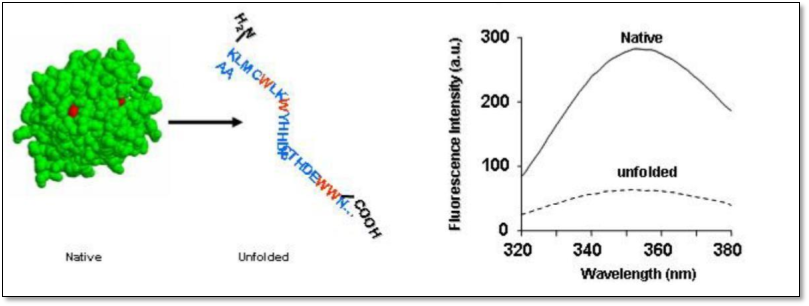
INTRINSIC PROTEIN FLUORESCENCE
- The amino acid tryptophan has the strongest fluorescence quantum yield of the amino acids found in proteins. The rest either do not fluoresce or fluoresce weakly. Therefore “Intrinsic Protein Fluorescence” usually refers to the fluorescence emission of the tryptophan amino acids.
- In a hydrophobic environment (buried within the core of the protein), Tyr and Trp have a high quantum yield and therefore a high fluorescence intensity. In contrast in a hydrophilic environment (exposed to solvent) their quantum yield decreases leading to low fluorescence intensity.
- For Trp residue, there is strong stoke shifts dependent on the solvent, meaning that the maximum emission wavelength of Trp will differ depending on the Trp environment.
FLUORESCENCE SPECTROMETER

- High resolution (1.0 nm)
- High scan speed up to 24,000 nm/min
- Sensitivity, high performance R928 PMT detector combined with PerkinElmer optics ensures optimum performance across the spectrum.
- Broad excitation and emission wavelength range up to 900 nm
- For samples at risk for photo-bleaching the FL 6500 provides accurate and high quality results with a user controllable pulsed excitation source.
- Superior wavelength accuracy and reproducibility for right first time results
TERTIARY STRUCTURE ANALYSIS BY FLD AT CBA
| Tertiary structure analysis by Fluorescence spectroscopy | |
| Sample requirement | Amount- 1 ml-3 ml (0.5-1 mg/ml)
Formulation buffer- 5 ml |
| Deliverables | FLD Spectra of at least two concentrations, Overlay FLD spectra with Tryptophan |
| Information required from client | • Concentration
• Buffer composition (in case of formulation) Note: Buffer composition that may quench or alter the fluorescence of a fluorophore, may interfere in assessing the Fluorescence emission properties. |
